Bacterial phosphate metabolism and its application to phosphorus recovery and industrial bioprocesses
| INTRODUCTION | |
| PI METABOLISM IN BACTERIA | |
| Pi RECOVERY FROM WASTE SLUDGES | |
| PHOSPHORUS RECYCLING | |
| DEVELOPMENT OF POLYP-DEPENDENT BIOPROCESS | |
| CONCLUSIONS | |
| ACKNOWLEDGEMENTS |
Phosphorus (P) is an essential constituent of all living organisms. It is present in nucleic acids, phospholipids, lipopolysaccharides, and various cytoplasmic solutes. However, global reserves of high-quality rock phosphate are limited, and they are being consumed rapidly (1). Because P is mostly used in fertilizers, P recovery and recycling are of considerable importance for sustaining profitable agricultural production in the long term (2). On the other hand, increased input of inorganic phosphate (Pi) to lakes, bays, and other surface waters can cause nuisance phytoplankton growth (3). Considerable attention has been paid to an efficient means of Pi removal from wastewater (4, 5, 6). Enhanced biological phosphorus removal (EBPR) has become a well-established process and is currently applied in many full-scale wastewater treatment processes (6). Waste sludge from an EBPR process contains large amounts of P; therefore, it cannot be disposed of into the environment without further treatment. If a sound recycling strategy is developed and applied, the waste sludge could be used as a viable source of raw material for the fertilizer industry.
Many microorganisms accumulate excess Pi in the form of polyphosphate (polyP) (7, 8). Biologically synthesized polyP is a linear polymer of Pi with a chain length of up to 1000 residues or more. PolyP can serve as a P source for the biosynthesis of nucleic acids and phospholipids under Pi starvation conditions (9). Although the biological roles of polyP are not fully understood, polyP is likely to function as a Pi reservoir with osmotic advantages. Activated sludge processes, which are commonly used for treating wastewaters, are very effective in removing organic pollutants, but they remove Pi relatively poorly. Municipal wastewaters are relatively deficient in carbon (6). This limits Pi removal by activated sludge whose P content is typically 1 to 2% on a dry weight basis. In EBPR processes, however, sludge microorganisms can accumulate Pi in excess of their growth requirement. They release Pi in the anaerobic tank but remove more Pi than that released in the anaerobic tank. A large portion of the Pi incorporated in the microorganisms is accumulated in the form of polyP. Consequently, the process performance relies primarily on the ability of sludge bacteria to accumulate polyP.
In this review, we will summarize our current knowledge on Pi metabolism in bacteria, highlighting molecular mechanisms of polyP accumulation. A better understanding of polyP accumulation in bacteria may be of help to further improve the biological Pi removal
from wastewater. Our efforts to genetically improve the ability of bacteria to accumulate polyP and to screen polyP-accumulating mutants from sludge bacteria will be briefly presented. Although the use of genetically modified microorganisms is practically infeasible for Pi removal, this allows us to understand the rate-limiting step of Pi removal by bacteria. In the second part, we will describe a simple method for releasing polyP and recovering P in a reusable form from waste sludge. We will show our recent work on P reuse as a raw material for the fertilizer industry. Finally, we will highlight the recent development of bioprocesses for the expanded use of polyP in the production of value-added chemicals.
PI METABOLISM IN BACTERIA
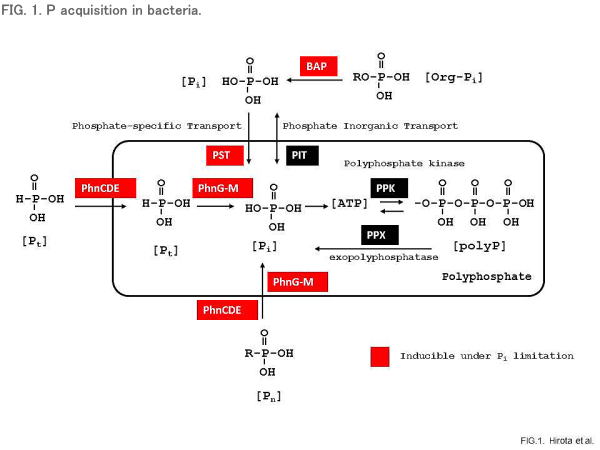
Phosphorus acquisition in bacteria
Bacteria use Pi as the preferred P source (Fig. 1). When Pi is available in excess, Pi is taken up by the Pi inorganic transport (Pit) system that is expressed constitutively (7). Under these conditions, bacteria can even store excess Pi in the form of polyP (9). Bacteria have evolved complex systems to survive under Pi starvation conditions. Under conditions of Pi starvation, the Pi-specific transport (Pst) system is turned on, and this system serves as a major scavenger of Pi residues. The Pst system of Escherichia coli is a periplasmic protein-dependent transporter similar to those of histidine, maltose, and ribose (10). These transporters belong to the superfamily of ATP-binding cassette (ABC) transporters. The Pst system of E. coli comprises four distinct subunits encoded by the pstS, pstA, pstB, and pstC genes (10). These genes, together with the phoU gene, form the pst operon and are involved in the regulation of the pho regulon.
Bacteria use organophosphates (Pi esters), inorganic phosphite (Pt), and phosphonates (Pn) as alternative P sources, when Pi is not available (11). Since most Pi esters are not transportable, Pi must be freed from organic combination before being taken up. This is accomplished through hydrolytic cleavage catalysed by a variety of enzymes including bacterial alkaline phosphatase (BAP) that is made at high levels under conditions of Pi starvation. Pn compounds are a large class of organophosphorus molecules that have direct carbon-phosphorus bonds in place of the more familiar carbon-oxygen-phosphorus ester bond. Utilization of Pn compounds as a P source requires cleavage of the carbon-phosphorus bond by C-P lyases (12). The genes encoding C-P lyase, together with a Pn transporter, are present in the phn gene cluster, which consists of 17 genes (12, 13). Several marine cyanobacteria can use Pn via a Phn pathway for their growth on the surface of an oligotrophic sea where extremely low Pi but a considerable amount of Pn is present (14). Pt seems to be enzymatically oxidized to Pi before being used as a P source. These enzymatic activities are also induced by Pi starvation stress (12, 13). Additionally, motile bacteria can also exhibit chemotactic responses toward Pi (Pi taxis) for their survival during Pi starvation (15,16). Pi taxis presumably gives bacteria a further selective advantage in the natural environment where nutrient concentrations change rapidly and unexpectedly.
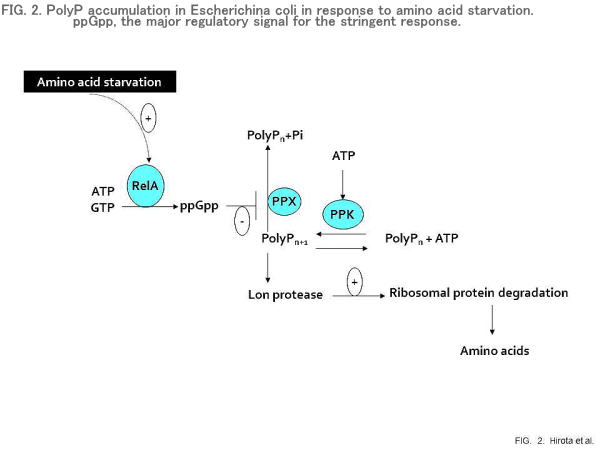
PolyP accumulation under amino acid starvation
The enzyme responsible for polyP biosynthesis is polyP kinase (PPK), which polymerizes the terminal Pi of ATP into polyP in a freely reversible reaction (17). The dimerization of PPK is crucial for the synthesis of polyP (forward reaction) and ATP (reverse reaction) (18). The crystal structure of E. coli PPK also revealed that PPK forms a dimer (19). The utilization and degradation of polyP is catalyzed by polyPases, including an exopolyPase (PPX), and several polyP-specific kinases, including polyP-glucokinase and polyP-fructokinase (9). The genes encoding PPK and PPX have been cloned from E. coli (20, 21). Curiously, however, E. coli does not accumulate appreciable amounts of polyP, despite its possession of PPK. Both PPK and PPX seem to be expressed constitutively. PolyP accumulation is not induced by Pi starvation stress in E. coli.
Many bacteria accumulate polyP under conditions of nutritional imbalance unfavourable for growth (8). This phenomenon has been called “Pi luxury uptake” in the studies of biological Pi removal (5). PolyP accumulation in E. coli takes place as a result of amino acid starvation (stringent response) (22). In E. coli, ppGpp, which is the major regulatory signal for the stringent response, inhibits PPX activity without affecting PPK activity (Fig. 2). This allows E. coli to accumulate polyP in response to amino acid starvation. We previously found that an E. coli mutant deficient in PPK failed to increase protein turnover and showed an extended lag in growth when shifted from a nutrient-rich to a nutrient-poor medium (nutritional downshift) (23). E. coli mutants lacking Lon protease also produce the same phenotype as ppk mutants. PolyP forms a complex with Lon, which enables the protease to degrade free ribosomal proteins. The interaction of ribosomal proteins with polyP seems necessary for the stimulation of Lon-mediated proteolysis (24, 25). Namely, the polyP-Lon complex can function as an adaptor molecule to capture substrates that can bind to polyP. This finding indicates a possible mechanism for “Pi luxury uptake” at the molecular level.
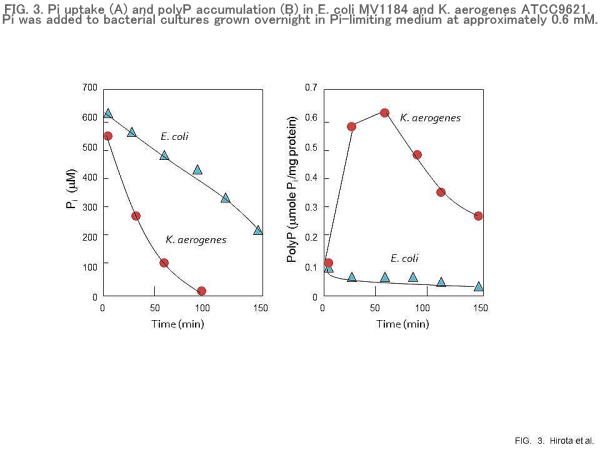
PolyP overplus in Klebsiella aerogenes
Many bacteria can exhibit rapid and extensive polyP accumulation, called “polyP overplus”, when Pi is added to cells previously subjected to Pi starvation stress (8). Obviously, E. coli is not a good system for investigating “polyP overplus”, because it is unable to accumulate polyP in response to Pi starvation stress. K. aerogenes (formerly named Aerobacter aerogenes) has been known to exhibit “polyP overplus” (Fig. 3). To identify the genes responsible for “polyP overplus”, we cloned the ppk gene from K. aerogenes ATCC9621 (26). The predicted product of the ppk gene shared 93% identical amino acids with E. coli PPK protein. Like the E. coli ppk-ppx operon, the ppx gene existed immediately downstream of the ppk gene. A pho box, the consensus sequence shared by the pho promoters, existed in the promoter region of the K. aerogenes ppk-ppx operon, suggesting that the ppk and ppx genes were involved in the regulation of the pho regulon (26).
Expression of multicopy lacZ fusion of the K. aerogenes ppk-ppx promoter in E. coli has been shown to increase under conditions of Pi limitation. However, if the K. aerogenes ppx gene is cotranscribed with the ppk gene, the question then arises concerning the mechanism by which K. aerogenes can show “polyP overplus”. To answer this question, the PPK and PPX activities were determined before and after “polyP overplus” took place (26). As expected, the PPK activity increased in response to Pi starvation and decreased upon addition of Pi. However, unlike PPK, the PPX activity did not increase under conditions of Pi starvation. Surprisingly, the PPX activity significantly decreased upon addition of Pi (26). Although the mechanism for controlling the PPX activity is unknown, both increased polyP synthesis and decreased polyP degradation are likely responsible for “polyP overplus” in K. aerogenes.
Rate-limiting step for polyP accumulation
As mentioned above, E. coli does not accumulate appreciable amounts of polyP, especially those of the high-molecular-weight types, despite its possession of PPK activity (20). Since the kinetics of bacterial polyP accumulation are not fully understood, it is unclear what limits polyP accumulation in E. coli. For a better understanding of polyP accumulation kinetics, Pi uptake experiments were carried out using E. coli MV1184 and its recombinant derivatives (4). Cells were grown in a complex broth and used for Pi uptake experiments in a minimal medium without being subjected to Pi starvation. Growth of recombinant strains was almost equivalent to that of the parental MV1184 strain. E. coli recombinants containing pBC29 (ppk) removed twice as much Pi from the medium than the parental MV1184 strain did. Strain MV1184 bearing pEP02.2 (pst operon) removed more Pi from the medium than MV1184 (pBC29) did. The P content of MV1184 (pEP02.2) was approximately twice that of MV1184 (pBC29). This finding suggests that Pi transport across the cell membrane is a rate-limiting step for polyP accumulation in E. coli under conditions of Pi excess.
Considerable changes in growth and Pi uptake were observed when pBC29 (ppk) and pEP02.2 (pst operon) were simultaneously introduced into strain MV1184. Even though the growth of this recombinant was severely limited in the minimal medium, the recombinant could remove approximately threefold more Pi than the parental strain. As a result, the P content of this recombinant reached a maximum of 16 % (48 % as Pi) on a dry weight basis. This value was approximately ten fold higher than that of the parental strain. The fractionation of cellular P revealed that acid-soluble and acid-insoluble polyPs accounted for approximately 65% of the total cellular P of MV1184 (pBC29 and pEP02.2). However, the disruption of PPX activity was not important for further improving the ability of E. coli to accumulate polyP (4).
When E. coli recombinants accumulated high levels of polyP, they released polyP into the medium (27). The release of polyP was confirmed by high-resolution [31P]NMR spectroscopy (27). PolyP release is likely to be the mechanism by which a further increase in cellular P is limited. The rate of polyP release, estimated from the increase in the concentration of acid-labile P (1 N HCl for 7 min at 100°C) in the culture supernatant, was dependent on that of Pi uptake. No polyP release was observed after the cells completely removed Pi from the medium, but it resumed soon after Pi was added to the medium. Although the mechanism of polyP release is unclear, a surface pool of polyP, which could be readily released into the medium, was detected by [31P] nuclear magnetic resonance (NMR) spectroscopy (27).
Screening of polyP-accumulating mutants
E. coli mutants that are capable of accumulating high levels of polyP can be selected by N-methyl-N'-vitro- N-nitrosoguanidine (NTG) mutagenesis (28, 29). The polyP accumulation in the mutants was ascribed to a mutation of the phoU gene that encodes a negative regulator of the pho regulon. Nucleotide sequence analysis showed that the 29th codon of the phoU gene was changed from a glycine to an aspartic acid in the polyP-accumulating mutant (28). In addition to the constitutive expression of the Pi-specific transport system, elevated levels of intracellular polyamines in the phoU mutants may contribute to the high levels of polyP (30). The polyamines are likely to stabilize polyP granules. Since the phoU mutants express alkaline phosphatase constitutively, they can be easily screened as blue-colored colonies on Pi excess agar plates containing 5-bromo-4-chloro-3-indolyl- phosphate (X-Pi).
Using this technique, we screened Pseudomonas putida and Acinetobacter sp. mutants that were able to accumulate high levels of polyP (29). A P. putida mutant, designated as MY11-41, accumulated approximately 15-fold more polyP than the parental strain. The total P content of MY11-41 reached approximately 9% on a dry weight basis (27% as Pi). Similarly, an Acinetobacter sp. mutant, designated as K3-6, accumulated 60-fold more polyP than the parental strain. Large polyP granules (PPGs) were detected in these mutants under a fluorescence microscope after staining with 4',6-diamidino-2-phenylindole (DAPI). The P. putida and Acinetobacter sp. mutants grew well in synthetic wastewater and could remove Pi effectively (29). This technique is likely to be useful for the screening of polyP-accumulating mutants from sludge bacteria without using recombinant DNA techniques.
Microbial aspects of EBPR process
EBPR primarily relies on the ability of sludge microorganisms to accumulate polyP. Sludge microorganisms release Pi in the anaerobic tank but remove more Pi in the subsequent aerobic tank than they released in the anaerobic tank (6,31). Although the detailed mechanism remains unclear, the alternate anaerobic and aerobic cycles are likely to help sludge microorganisms accumulate excess Pi in the form of polyP. During the anaerobic phase, sludge microorganisms take up organic compounds from wastewater and accumulate storage biopolymers (mainly PHAs and glycogen). PolyP is used as the energy source for this process, and the resultant Pi is released from the sludge. PHA molecules serve as energy and carbon sources for uptake of Pi during the subsequent aerobic stage (32,33). Glycogen, which usually serves as a regulator of redox balance in cells, provides additional energy, helping polyP-accumulating microorganisms to take up organic substances under anaerobic conditions (32).
Microbiologists have been studying the primary players in EBPR since its introduction in municipal wastewater treatment plants over 30 years ago (34,35). The bacterial genus Acinetobacter was incorrectly assumed to be primarily responsible for EBPR on the basis of cultivation studies (36). Recently, culture-independent methods have allowed the identification of Accumulibacter phosphatis, a member of the order Rhodocyclales, as the principal microorganism in acetate-fed EBPR (36,37). A. phosphatis has yet to be grown in axenic culture despite continuing efforts, but it has been enriched in up to 85% of the community in lab-scale bioreactors. Metagenome analysis has recently been applied to the microbial community in EBPR processes (38). This technique will be of help in further understanding of the mechanism underlying EBPR .
Pi RECOVERY FROM WASTE SLUDGES
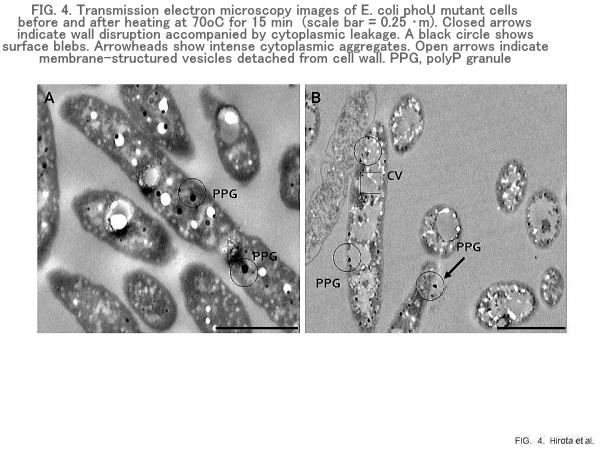
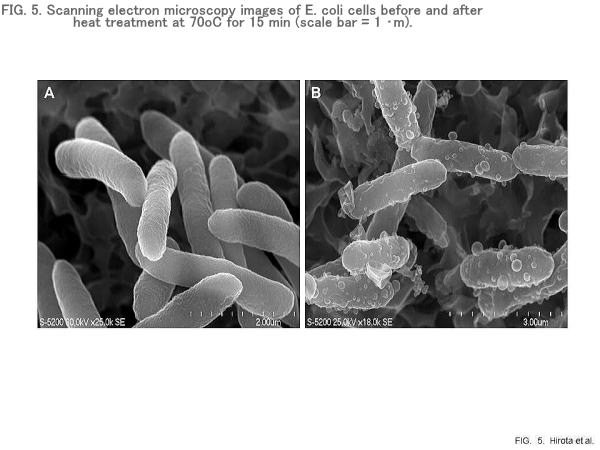
Heat-induced polyP release from E. coli
E. coli phoU mutants contain PPGs of various sizes in the cytoplasm (Fig. 4). High levels of P were detected in cell areas containing the PPGs by energy-dispersive X-ray (EDX) analysis. The polyP was released from the E. coli cells by heating at 70oC. Heating of the cells at 70oC also caused the formation of blebs of various sizes on the cell surface (Fig. 5). Although considerable irregularity in the shape of the bacterial cells was observed, there was little or no evidence of cell lysis. Cytoplasmic leakage was visible near the heated cells, and considerable amounts of P were detected in the cytoplasmic leakage.
Ultrastructural analysis of PPGs in response to heating at 70oC was performed using TEM and EDX analyses. Before heat treatment, PPGs were randomly distributed in the cytoplasm (Fig. 4). Occasionally, large PPGs were visible in the untreated cells. Soon after the start of heating, cell voids resulting from plasmolysis and cytoplasmic aggregations became visible. Although large cell voids were formed in the cytoplasm, no significant P was detectable in cytoplasmic voids. PPGs were also perceivable around the surface of the heated cells. These PPGs were likely present, at least in part, in the cytoplasmic leakage. Obviously, the sizes of PPGs became significantly smaller than detected before heating, suggesting the occurrence of PPG degradation in the cytoplasm. Although small PPGs were also detectable on the surface of cell voids, no significant P was detected in the cell voids. These results suggest that PPGs per se, in part, leaked from heated cells through the openings of the ruptured cell envelope. Heating of bacteria at 70oC led to significant structural alterations such as plasmolysis, cytoplasmic aggregation, cell void formation, wall disruption, and cytoplasmic leakage. Heating also caused blebbing and vesiculation of the cell surface. These events are likely to cause the leakage of PPGs from the heated cells.
Release of polyP from activated sludge
Heating at 70oC was also effective in the release of polyP from activated sludge (39). In laboratory experiments, activated sludge samples were taken from a bench-scale biological Pi removal process, and they were heated at temperatures ranging from 50-90oC for 1 h. Fluorescence microscopy analysis revealed that DAPI-stained polyPs were released into the liquid phase from activated sludge by heat treatment. Bluish-white-colored DNA molecules remained nearly unchanged inside the activated sludge flocs, suggesting that polyP might be specifically released from activated sludge during heat treatment. The rate and extent of polyP release were clearly dependent on temperature. For example, nearly all the polyP could be released in 10 min at 90oC, 30 min at 80oC, and 60 min at 70oC (39). Total P (T-P) increased essentially in parallel with polyP content, indicating that the released P was almost entirely polyP. Approximately 87% of cellular P was released into the liquid phase by heating at 70-90oC. However, both the rate and extent of polyP release markedly decreased at temperatures lower than 50oC. Degradation of the released polyP was observed in the liquid phase. The rate of polyP degradation was also dependent on the heating temperature. Approximately 20% of polyP was degraded to Pi at 70oC in the liquid phase, while more than 60% of polyP disappeared at 90oC. Heat treatment did not decompose activated sludge flocs and did not significantly change their settleability.
The chain length of released polyP was determined by polyacrylamide gel electrophoresis (PAGE) (39). 32Pi-labelled polyP could be detected in the liquid phase soon after the start of heating at 70oC. Initially, the released polyP had a chain length of approximately 100-200 Pi residues. However, the chain length of polyP gradually decreased because of polyP hydrolysis. After 2 h of heat treatment, the fraction of polyP longer than 100 Pi residues disappeared. In addition, a P compound was found to migrate faster than Pi in PAGE. High-pressure liquid chromatography (HPLC) analysis results showed this compound as trimetaphosphate (cyclic tripolyphosphate). The released polyP was almost completely degraded to Pi when it was treated with exopolyPase.
Released polyP is easily precipitated by the addition of CaCl2 at room temperature without adjusting pH. Floc formation was visible soon after the addition of 50 mM CaCl2 to the supernatant of a sample heated at 70oC for 1 h. Addition of CaCl2 precipitated about 65% of T-P during the first 30 min and approximately 75% of T-P by 2 h. The calcium-phosphorus (Ca-P) precipitation was significantly influenced by the concentration of polyP in the supernatant. After polyP had been degraded to Pi, Ca-P precipitation was negligible, unless pH was adjusted to 10 or higher. Trimetaphosphate was not precipitated with CaCl2 even at pH 10. Releasing polyP, but not Pi, can offer a distinct advantage in recovering P from the liquid phase. Element analyses revealed that the precipitated materials contained about 16 and 18% of their dry weight as P and Ca, respectively. These values were greater than those of natural rock phosphate that typically contains 8 to 13% P and 26 to 33% Ca. The precipitated materials also contained about 4 % of their dry weight as carbon.
PolyP-rich activated sludge can release Pi, but not polyP, back into the solution when it is subjected to anaerobiosis (40). When compared with the anaerobic release of Pi, the heating method showed much better performance for releasing P from activated sludge. The addition of a readily decomposable organic acid, for example acetate, can significantly increase the anaerobic release of Pi. However, in the absence of readily decomposable organic acids, the release of Pi becomes negligible even under anaerobic conditions. An obvious disadvantage of the heating technique is the additional cost required for heating waste sludge. Methane gas, which is generated in wastewater treatment plants, can be used for heating sludge to release P. The use of a heat exchanger may save the thermal energy effectively. The heating technique has the potential to produce a raw material containing Pi at levels similar to those of natural rock phosphate. The Pi-rich material recovered from activated sludge has been designated as “biophosphorite” (39).
Feasibility tests of “Heatphos” process
The feasibility of the P-recovery process (designated as “Heatphos”) was examined at pilot plant scale (41). A pilot test plant was built at a municipal wastewater treatment facility located in Kobe City, Japan. The pilot plant consisted of an EBPR process for biological Pi removal and a process for Pi recovery from waste sludge. The pilot plant influent was obtained from the primary clarifier of a full-scale wastewater treatment facility and introduced into the anaerobic tank of the pilot plant at a rate of approximately 100 m3/d. The volumes of the anaerobic and aerobic tanks were 9 and 23 m3, respectively. The level of mixed liquor suspended solids (MLSS) in the aerobic tank was maintained at about 2,000 mg/l, while that of the return sludge was approximately 6,000 mg/l. About 50 % of the thickened sludge was returned from the settling tank to the anaerobic tank. The sludge retention time (SRT) was about 4.5 d. Waste sludge was generated at a rate of approximately 10-20 kg MLSS/d. The waste sludge was heated at 70-90°C for about 1 h in a 1 m3-capacity heating tank. P-rich liquid was separated from sludge solids in a flotation tank, and P was then precipitated with CaCl2.
In the feasility test, approximately 90-95% of influent T-P could be removed from municipal sewage throughout the one-year operating period. The T-P content of activated sludge in the aeration tank reached approximately 3.5 to 4.5% on a dry weight basis. In general, sludge microorganisms can accumulate about 1 % of their cell dry weight as P in the form of nucleic acids and phospholipids. The polyP content reached 2.0-3.0% about 2 months after the start of operation. This showed that about 50-70% of influent T-P was stored in the form of polyP by activated sludge. Approximately 50 to 60% of cellular T-P could be released from waste sludge by treatment at 70oC for 1 h. P compounds contained in molecules such as nucleic acids and phospholipids cannot be released from activated sludge simply by heating at 70oC for about one h (39). The P content of waste sludge from the pilot plant was as high as 4% on a dry weight basis. This suggests that at most 75 % of T-P could be released from the waste sludge by heating. Taken together, the performance of the pilot plant was satisfactory for P release from polyP-rich waste sludge.
Chemical analysis results showed a large amount of ferric salts that formed insoluble Pi precipitates outside the sludge solids. To investigate the effect of ferric ions on polyP release, bench-scale experiments were carried out using synthetic wastewater supplemented with either FeCl2 or Fe2(SO4)3. PolyP release was strongly inhibited by the presence of ferric salts in the synthetic wastewater. Conversely, polyP release was significantly improved by the addition of citrate as a chelating agent to the sludge samples before heat treatment. In bench-scale experiments, about 65% of the released P, which was mainly polyP, could be precipitated with CaCl2 at a Ca:P ratio of 2:1 without pH adjustment (41). However, in the pilot tests, only about 3% of T-P was precipitated under these conditions. The reason for the difference in % P recovery between the pilot-scale and bench-scale experiments was attributed to the form of P compounds released into the liquid phase by heat treatment. PolyP precipitates with Ca at neutral pH more efficiently than Pi, because the pKa value of polyP is lower than that of Pi. In the pilot tests, approximately 70% of the released P was detected as Pi When pH was adjusted to11, 80 % of T-P was precipitated with CaCl2 even at the stoichiometric Ca:P ratio of 1:1. Overall, approximately 40% of T-P could be recovered from municipal sewage in the pilot tests.
A full-scale test plant was installed at a wastewater treatment facility in Fukuyama City, Japan (Fig. 6). The influent T-P to the full-scale plant ranged from about 40 to 60 mg-P/L throughout the one-year period of operation. After the start-up period of about 30 d, % removal of T-P increased dramatically, and the effluent T-P decreased down to less than 1 mg-P/L. After then, an average P removal of 90% was achieved throughout the operating period. PolyP-rich waste sludge was processed in the full-scale test plant at a rate of approximately 360 kg/d, which was twenty times higher than that of the pilot-scale test plant. P-rich waste sludge from the wastewater treatment facility was first stored in a storage tank of the full-scale test plant. Then, the sludge was condensed approximately three fold in a sludge flotation tank. After being passed through a heat exchanger, the condensed sludge was heated at 70oC for one h in a heating tank in continuous mode. To recover heat energy, the heated sludge was once again passed through the heat exchanger and cooled to atmospheric temperature. After centrifugation, the released P compounds were precipitated by adding CaCl2 to the supernatant. The full-scale test plant could generate “biophosphorite” from waste sludge at a rate of approximately 70 kg/d (10 kgP/d).

Other techniques for P recovery
Magnesium ammonium phosphate hexahydrate (MgNH4PO4.6H2O), commonly known as struvite (42), is a P compound recovered from wastewater. Struvite often forms in anaerobic sludge digestion (ASD) processes where high concentrations of Pi and ammonium are present (43,44). Effective formation of struvite occurs at low concentrations of suspended solids, pH higher than 7.5, and the molecular ratio of 1(Mg2+):1(NH4+):1(PO43-). Recently, struvite crystals have been produced from the filtrate of ASD slurry by adding magnesium hydroxide at pH 8.2?8.8. A retention time of 10 days allowed the formation of struvite pellets of 0.5?1.0 mm diameter. Struvite has the potential to be used as fertilizer. This fertilizer can slowly release P during the growing season (43, 44, 45). However, it remains unclear if struvite is commercially profitable for the fertilizer industry. Since some plants require large amounts of Pi at their initial growth stage, more water-soluble Pi may need to be mixed with struvite to make an effective P fertilizer. The direct use of nonsoluble Pi as a fertilizer requires an effective and cheap means of solubilization. This problem may be solved with the use of Pi-solubilizing microorganisms (46, 47).
A macroporous (approximately 100 nm diameter) fibrous structure of TiO2 removes large quantities of Pi as hydroxyapatite (48). Another option is to use a strongly adsorbing filter material that retains P efficiently. Blast furnace slag showed a high P sorption capacity and was used for abiotic sorption of wastewater P. It can adsorb P in the presence of Ca, forming calcium hydroxyapatite (49). High-performance packed columns have also been developed for Pi removal using zirconium particles (50) and a high-speed adsorbent having unique porous structure (51). A seed crystal material made of calcium silicate hydrate (tobermorite crystals) was used for the crystallization of hydroxyapatite that can be incorporated into soil and serve as a good plant fertilizer (52). Alternatively, sludge incineration ash from excess sludge after P removal is a source of Pi, if iron and aluminium are not excessively used for Pi precipitation in the treatment plant. If large amounts of heavy metals such as Cu and Zn are present in the ash, the use of recovered P as fertilizer is undesirable (53).
PHOSPHORUS RECYCLING
National phosphorus metabolism
A modification of the Substance Flows Analysis tool from Industrial Ecology has been applied to track global P flows (2). Material flow analysis has also been conducted for the national P metabolism in Japan (54). The domestic flow of P materials in Japan was analyzed by examining various statistical data for FY2002. Since national P metabolism has not been subject to full monitoring, the material flow is restricted by current data availability, inconsistency, and uncertainty. Additionally, P negligibly exists as a pure element and circulates in society in a state of relatively low concentration (54). The domestic P flow was not estimated by assuming a steady state in associated individual sectors of production and consumption.
There is no phosphate rock production in Japan. Nearly all the P consumed domestically is imported from China, South Africa, Morocco, and Jordan. The total P input into Japan was estimated to be 764 thousand metric tonnes of P per year (kmtP/Y). Phosphate rock import was 110 kmtP/Y. The amount of P imported as fertilizers was 141 kmtP/Y, while that involved in food and feed was 173 kmtP/Y. Input P flow as chemical reagents was 155 kmtP/Y. In addition, approximately 157 kmtP/Y was imported as raw minerals for the steel industry. In the domestic P flow, the largest demand arises in the fertilizer industry. The total amount of P used in fertilizers was approximately 400 kmtP/Y. Although a portion of this P circulated in the agriculture and livestock sectors in the form of feed and livestock biomass, most was lost to the environment, including soils and rivers, or in wastes. In particular, the soils used for agricultural purposes were a tremendous P sink. Approximately 356 kmtP/Y or about 50% of the annual national P input was accumulated in the cultivated soils. The cultivated soils also served as an active source of P leakage into surface waters. Approximately 10% of the pool for accumulation was loaded into surface waters.
The P inflow in the chemical industry was about 265 kmP/Y. More than 80% of the P was used in fertilizer manufacturing. The P input to the iron and steel industries was estimated to be 104 kmtP/Y, approximately 90% of which was concentrated in steelmaking slag. Release of P in waste sludge from wastewater treatment facilities was approximately 43 kmtP/Y. Together, the amount of P in steelmaking slag and waste sludge exceeded the total amount of P imported as phosphate rock. Since steelmaking slag and waste sludge contain relatively high concentrations of P, they are good targets to recover P for the immediate and potential future of agriculture and industry.
Technologies for using recovered P
Utilization of phosphate rock is primarily via fertilizer application. A wide variety of fertilizers, including straight phosphate fertilizers, compound fertilizers, and mixed fertilizers, are produced and put into the global market. The diversity of fertilizers is of importance for facilitating site-specific application, which takes account of factors such as soil type, the requirements of the crop, and weather conditions, thus making it possible to achieve optimal plant nutrition and minimal environmental impact. The use of recovered P has been examined for the production of straight phosphate fertilizers (by-product phosphate fertilizer and amended phosphate fertilizer) (55). By-product phosphate fertilizer needs to contain at least 15% citric acid-soluble phosphate (C-P2O5) as P2O5. In addition, the total nitrogen (T-N) content must be lower than 1%. Amended phosphate fertilizer is made by the phosphoric acid acidification of by-product phosphate fertilizer. This straight phosphate fertilizer needs to contain at least 35% C-P2O5 and 16% water-soluble phosphate (W-P2O5). “Biophosphorite” typically contained 31% C-P2O5, 15% organics (ignition loss), and 1-2% of T-N on a dry weight basis. To make by-product phosphate fertilizer, biophosphorite was further processed by calcination at 550oC for 2 h. The calcined product typically contained 28% C-P2O5 and no significant amounts of T-N and organics. Calcium silicate hydrate (CSH) was also used as a seed material to stimulate the crystallization of calcium phosphate. CSH leaches calcium ions, increases the pH in the solution near the solid surface, and provides available calcium for the formation of calcium phosphates. The CSH seed was found to considerably increase calcium phosphate precipitation. The Ca-P precipitates typically contained 15% C-P2O5 when the CSH seed was used at the stoichiometric Ca:P ratio of 2:1. Importantly, the resulting precipitates contained T-N of 1% or less, indicating that it can be directly used as by-product phosphate fertilizer.
It is known that mineral elements such as Fe, Mg, Al, Na, and K restrict the extraction of Pi from natural rock phosphate. Mineral concentrations lower than 2% were preferred in calcined biophosphorite (55). To overcome this problem, calcined biophosphorite was used in the mixture with natural rock phosphate. Purified Pi (75% w/v) was produced by the mixture of 10% calcined biophosphorite and 90% natural rock phosphate. Impure phosphoric acid was also made from the mixture of 20% biophosphorite and 80% natural rock phosphate. The impure phosphoric acid was utilized to produce amended phosphate fertilizer. As a result, it was possible to replace approximately 50% of the natural rock phosphate with calcined biophosphorite as a raw material for the production of amended phosphate fertilizer.
DEVELOPMENT OF POLYP-DEPENDENT BIOPROCESS
Strictly polyP-dependent glucokinase
PolyP is readily formed by dehydration of Pi and found abundantly in volcanic condensates and deep-oceanic steam vents (56). Hence, ancient organisms may have utilized polyP instead of ATP in their metabolic reactions. Glucokinases that use ATP as the sole phosphoryl donor to catalyze the phosphorylation of glucose (ATP-GKs) are present in all contemporary organisms examined (57). Bifunctional glucokinase (polyP/ATP-GK), which utilizes polyP or ATP as the phosphoryl donor to phosphorylate glucose, was first found in Mycobacterium phlei and then in many other bacteria including Corynebacterium diphtheriae, Mycobacterium tuberculosis, and Propionibacterium shermanii (58, 59, 60). The polyP/ATP-GK of M. tuberculosis also utilizes GTP, UTP, and CTP as phosphoryl donors (59). PolyP is recognized as one of the earliest biopolymers and is most likely a prominent precursor in prebiotic evolution. It has been hypothesized that glucose phosphorylation was originally mediated by polyP and that when ATP became available in the environment, a transition was made to utilize the latter phosphoryl donor.
Microlunatus phosphovorus strain NM-1 is a gram-positive, coccus-shaped, nonspore-forming bacterium (61). Strain NM-1, which was originally isolated from an EBPR process, accumulates large amounts of polyP (approximately 48% of its dry weight as Pi) in a glucose medium (62). The existence of strictly polyP-dependent glucokinase (polyP-GK) was discovered in M. phosphovorus strain NM-1 and it was purified 250-fold to homogeneity from this organism (62). The purified enzyme produced glucose-6-Pi from glucose and polyP. The glucose-6-Pi was identified by being oxidized to glucose-6-phosphonate with glucose-6-Pi dehydrogenase and NADP. The polyP-GK migrated as a 32-kDa protein on sodium dodecylsulfate (SDS)-PAGE, while it was fractionated as a 64-kDa protein by gel filtration, suggesting that it was a dimer. The polyP-GK could not phosphorylate glucose with 5-10 mM [γ-32P]-ATP or at the usual intracellular levels of ATP (62). The production of 32P-labeled glucose-6-Pi from 32P-polyP (0.6 µM as a polymer) was inhibited by 30% in the presence of 1.6 mM cold ATP. The polyP-GK could phosphorylate glucosamine at a rate similar to that of glucose. The polyP-GK activity required Mg2+and was inhibited by EDTA. The optimal temperature and pH were 30oC and 5.5, respectively.
After determining the N-terminal and internal amino acid sequences of the polyP-GK, the ppgk gene encoding polyP-GK was cloned from M. phosphovorus chromosomal DNA (62). The M. phosphovorus ppgk gene encoded a putative polypeptide of 266 amino acids with a calculated mass of 32 kDa. The polyP-GK was most closely related to the polyP/ATP-GK of M. tuberculosis. The deduced amino acid sequence of the polyP-GK showed 50% identity (65% similarity) to that of the M. tuberculosis polyP/ATP-GK. The polyP-GK contained regions that are homologous to common motifs interacting with the ATP molecule, which are conserved in the ATP- and polyP/ATP-GKs from different sources. In addition, the polyP-GK was found to possess a putative polyP-binding region that was homologous to that of the M. tuberculosis polyP/ATP-GK. The putative glucose-binding region proposed in the polyP/ATP-GK was conserved in polyP-GK.
A long-standing question was whether a strictly polyP-dependent glucokinase exists in contemporary organisms. The M. phosphovorus polyP-GK might be the hypothetical evolutionary origin of glucokinase. Interestingly, M. phosphovorus strain NM-1 was found to release Pi concomitantly with the degradation of intracellular polyP, when it took up glucose under anaerobic conditions. This evidence, together with the existence of polyP-GK, strongly suggests that polyP can be directly used to phosphorylate glucose in M. phosphovorus. When the M. phosphovorus cells were aerobically grown in a glucose medium with Pi excess, the cellular levels of polyP (mainly 100 to 200 Pi residues) reached over 5 mmol/g of cells in Pi equivalents, which far exceeded those observed with ATP (typically 7 ?mol/g cells). However, it is unlikely that ATP is directly used for the biosynthesis of polyP, because no PPK activity was detected with M. phosphovorus strain NM-1. The cellular environment of this unique bacterium may present a hypothetical ancient world in which polyP could be preferentially utilized in metabolic roles. The cost of ATP for use as an enzymatic phosphorylating agent on an industrial scale is prohibitive. The use of polyP as the phosphoryl donor could be a promising strategy in the development of cost-effective bioprocesses. The discovery of polyP-GK may open a new route for the application of polyP-utilizing enzymes to industrial bioprocesses.
PolyP-dependent ATP regeneration
Bacterial contamination in food has a deleterious effect on food quality. Therefore, it is of importance to develop a sensitive and reliable method for hygiene monitoring. ATP has been used as an indicator in detecting food residues as a nutrient source susceptible to bacterial contamination in swab monitoring by hazard analysis and critical control points (HACCP). The enzymatic determination of ATP using firefly luciferase is a well-established technique for detecting ATP (10-11 to 10-6 M). The principle of this method is based on the ATP dependence of the light emitting luciferase that catalyzes the oxidation of luciferin. However, swab monitoring is often hampered by the degradation of ATP in food residues to adenosine monophosphate (AMP) during heat treatment. Therefore, detecting not only ATP but also AMP in food samples has been proved to be effective for assessing bacterial contamination (63).
A sensitive method has been developed for the detection of AMP using polyP-ATP phosphotransferase (PPT) and adenylate kinase (ADK) in conjunction with firefly luciferase (63). The method was based on: (i) phosphorylation of AMP by PPT with polyP as the phosphoryl donor; (ii) conversion of two molecules of ADP to ATP and AMP by ADK, and (iii) detection of the resultant ATP by bioluminescence assay. This method used polyP in place of ATP for initiating the reactions. PPT was partially purified from A. johnsonii about 20-fold. About 80 % of AMP was converted to ATP by PPT and ADK after 120 min. AMP detection sensitivity was proportional to its concentration with a wide range from 10-7 to 10-4 M AMP (0.3 to 300 pmol per assay). Both AMP and ADP in various nutrient samples were converted to ATP by PPT and ADK with polyP, resulting in higher luminescence than that obtained without ATP conversion reaction. The detection of ATP+ADP+AMP in various nutrient sources increased the sensitivity by 5- to 177,000-fold, depending on the nutrient source, compared with that relying only on the amount of ATP (64). This result indicated that polyP could be used for sensitive hygiene monitoring. PolyP is a relatively inexpensive and stable substrate compared with the common phosphoryl donors such as acetyl phosphate and phosphoenol pyruvate. The bioluminescent enzymatic assay with concomitant use of PPT, ADK, and polyP, which results in long-lived luminescence, will ultimately contribute to the development of a sensitive, inexpensive, and reliable method for hygiene monitoring.
Thermostable PPK for industrial application
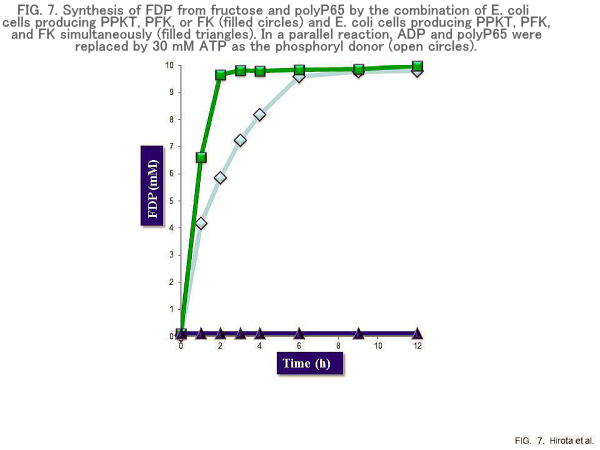
ATP is the most important biological phosphoryl donor and is required for many enzymatic reactions. The direct addition of ATP is not only expensive but also leads to the accumulation of inhibitory by-products, such as ADP or AMP (64). Furthermore, high concentrations of ATP inhibit enzymes such as phosphofructokinase (PFK) (64). These problems can be overcome by including an ATP-regenerating system in enzymatic reactions. ATP can be synthesized from polyP and ADP with PPK and also from AMP using PPK and PPT. The genus Thermus has thermostable enzymes that have enormous potential for many industrial applications. The production of thermostable enzymes in mesophilic hosts such as E. coli inactivates only host enzymes by a simple heat treatment, which reduces the production of by-products. This system was applied for the production of fructose 1,6-diphosphate (FDP), which can be used to reduce ischemic injury in the myocardium, brain, and kidney (64). A thermostable PPK (PPKT) from Thermus thermophilius HB27 was cloned into a vector plasmid. The resultant plasmid, pETPPKT, was introduced into E. coli. The E. coli recombinant was heated at 70oC for 10 min and used to synthesize ATP from external ADP and polyP65 (65 Pi residues). The extracellular accumulation of ATP was observed when the cells were incubated at 70oC (Fig. 7). When the heated cells were centrifuged, PPKT activity was detected in the cell pellet but not in the supernatant. More than 60% of the PPKT activity was retained even after a one-week incubation at 70°C. To allow the production of FDP in this system, the genes encoding fructokinase (FK) and phosphofructokinase (PFK) were also cloned from T. thermophilus HB27. The combined use of heat-treated E. coli recombinants producing FK, PFK, and PPKT, respectively, synthesized FDP at 70°C. When 30 mM ATP was used instead of polyP, no significant amount of FDP was produced. This may be ascribed to the fact that FK is perfectly inhibited by 10 mM ATP. ATP regeneration from polyP and ADP could minimize this inhibitory effect due to ATP (64).
An E. coli recombinant, which was capable of producing FK, PFK, and PPKT simultaneously, was constructed to increase the rate of FDP production. The heat-treated E. coli recombinant successfully synthesized FDP. The rate of FDP synthesis increased approximately twofold compared with that of the mixture of heat-treated E. coli recombinants separately producing FK, PFK, and PPKT. Essentially, all the fructose was converted to FDP within 3 h. The heat-treated E. coli producing PPKT can be used as an ATP regenerator for at least one week at 70°C. The biggest advantages of the present method are the stability of PPKT and the use of an inexpensive phosphagen, polyP, for ATP regeneration (64). Another ATP regeneration system with thermostable PPK from Thermosynechococcus elongates BP-1 (TePpk) has been used for the effective production of amino acid dipeptide (65,66). Thermostable ATP regeneration systems can be used as a biocatalytic platform to produce value-added chemicals.
CONCLUSIONS
Natural phosphate rock is a nonrenewable resource. Japan has no significant reserve of natural phosphate rock and is located far from the mineral source. This highlights the importance of transportation costs and the increasing need to move only high-grade ores containing P2O5 of 35% or more over long distances. The quality of natural rock phosphate is becoming more variable leading to lower P2O5 levels coupled with higher levels of impurities such as heavy metals and radionuclides. This leads to the consideration of alternative technologies where P may be derived from pre-existing materials such as sewage waste and steelmaking slag. If a P recovery process is applied to municipal sewage treatment facilities throughout Japan, the amount of natural rock phosphate that Japan has to import from foreign countries could be reduced by approximately 40%. A sustainable and safe strategy for P recycling will benefit not only industry but also society.
ACKNOWLEDGEMENTS
The research consortium for P recovery and recycling has been supported by the Research and Development Program for New Bio-industry Initiatives at the Bio-oriented Technology Research Advancement Institution (BRAIN).
References
| 1 | Abelson, P. H.: A potential phosphate crisis. Science, 283, 2015 (1999). |
| 2 | Cordell, D., Drangert, J., and White, S.: The story of phosphorus: Global food security and food for thought. Global Environ. Change, 19: 292-305 (2009). |
| 3 | Hammond, A. L.: Phosphate replacements: problems with the washday miracle. Science, 172, 361-363 (1971). |
| 4 | Kato, J., Yamada, K., Muramatsu, A., Hardoyo, and Ohtake, H.: Genetic improvement of Escherichia coli for enhanced biological removal of phosphate from wastewater. Appl. Environ. Microbiol., 59, 3744-3749 (1993). |
| 5 | Ohtake, H., Takahashi, K., Tsuzuki, Y., and Toda, K.: Uptake and release of phosphate by a pure culture of Acinetobacter carcoaceticus. Water Res., 19, 1587-1594 (1985). |
| 6 | Sedlak, R. I.: Phosphorus and nitrogen removal from municipal wastewater, 2nd ed. Lewis Publishers, New York, USA (1991). |
| 7 | Kulaev, I. S. and Vagabov, V. M.: Polyphosphate metabolism in micro-organisms. Adv. Microbiol. Physiol., 24, 83-171 (1983). |
| 8 | Harold, F. M.: Inorganic polyphosphates in biology: structure, metabolism, and function. Bacteriol. Rev., 30, 772-794 (1966). |
| 9 | Kornberg, A.: Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J. Bacteriol., 177, 491-496 (1995). |
| 10 | Wanner, B. L.: Molecular genetics of carbon-phosphorus bond cleavage in bacteria. Biodegradation, 5, 175-184 (1994). |
| 11 | Chen, C. M., Ye, Q. Z., Zhu, Z. M., Wanner, B. L., and Walsh, C. T.: Molecular biology of carbon-phosphorus bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and C-P lyase activity in Escherichia coli B. J. Biol. Chem., 265, 4461-4471 (1990). |
| 12 | Wackett, L. P., Shames, S. L., Venditti, C. P., and Walsh, C. T.: Bacterial carbon-phosphorus lyase: products, rates, and regulation of phosphonic and phosphinic acid metabolism. J. Bacteriol., 169, 710-717 (1987). |
| 13 | Metcalf, W. W. and Wanner, B. L.: Involvement of the Escherichia coli phn (psiD) gene cluster in assimilation of phosphorus in the form of phosphonates, phosphite, Pi esters, and Pi. J. Bacteriol., 173, 587-600 (1991). |
| 14 | Dyhrman, S. T., Chappell, P. D., Haley, S. T., Moffett, J. W., Orchard, E. D., Waterbury, J. B., and Webb, E. A.: Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature, 439, 68-71 (2006). |
| 15 | Kato, J., Ito, A., Nikata, T., and Ohtake, H.: Phosphate taxis in Pseudomonas aeruginosa. J. Bacteriol., 174, 5149-5151 (1992). |
| 16 | Kato, J., Kim, H., Takiguchi, N., Kuroda, A., and Ohtake, H.: Pseudomonas aeruginosa as a model microorganism for investigation of chemotactic behaviors in ecosystem. J. Biosc. Bioeng., 106, 1-7 (2008). |
| 17 | Ahn, K. and Kornberg, A.: Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. J. Biol. Chem., 265, 11734-11739 (1990). |
| 18 | Tzeng, C. M. and Kornberg, A.: The multiple activities of polyphosphate kinase of Escherichia coli and their subunit structure determined by radiation target analysis. J. Biol. Chem., 275, 3977-3983 (2000). |
| 19 | Zhu, Y., Huang, W., Lee, S. S., and Xu, W.: Crystal structure of a polyphosphate kinase and its implications for polyphosphate synthesis. EMBO Rep., 6, 681-687 (2005). |
| 20 | Akiyama, M., Crooke, E., and Kornberg, A.: The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. J. Biol. Chem., 267, 22556-22561 (1992). |
| 21 | Akiyama, M., Crooke, E., and Kornberg, A.: An exopolyphosphatase of Escherichia coli. The enzyme and its ppx gene in a polyphosphate operon. J. Biol. Chem., 268, 633-639 (1993). |
| 22 | Kuroda, A., Murphy, H., Cashel, M., and Kornberg, A.: Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem., 272, 21240-21243 (1997). |
| 23 | Kuroda, A., Tanaka, S., Ikeda, T., Kato, J., Takiguchi, N., and Ohtake, H.: Inorganic polyphosphate kinase is required to stimulate protein degradation and for adaptation to amino acid starvation in Escherichia coli. Proc. Natl. Acad. Sci. USA, 96, 14264-14269 (1999). |
| 24 | Kuroda, A., Nomura, K., Ohtomo, R., Kato, J., Ikeda, T., Takiguchi, N., Ohtake, H., and Kornberg, A.: Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science, 293, 705-708 (2001). |
| 25 | Nomura, K., Kato, J., Takiguchi, N., Ohtake, H., and Kuroda, A.: Effects of inorganic polyphosphate on the proteolytic and DNA-binding activities of Lon in Escherichia coli. J. Biol. Chem., 279, 34406-34410 (2004). |
| 26 | Kato, J., Yamamoto, T., Yamada, K., and Ohtake, H.: Cloning, sequence and characterization of the polyphosphate kinase-encoding gene (ppk) of Klebsiella aerogenes. Gene, 137, 237-242 (1993). |
| 27 | Hardoyo, Yamada, K., Shinjo, H., Kato, J., and Ohtake, H.: Production and release of polyphosphate by a genetically engineered strain of Escherichia coli. Appl. Environ. Microbiol., 60, 3485-3490 (1994). |
| 28 | Morohoshi, T., Maruo, T., Shirai, Y., Kato, J., Ikeda, T., Takiguchi, N., Ohtake, H., and Kuroda, A.: Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol., 68, 4107-4110 (2002). |
| 29 | Morohoshi, T., Yamashita, T., Kato, J., Ikeda, T., Takiguchi, N., Ohtake, H., and Kuroda, A.: A method for screening polyphosphate-accumulating mutants which remove phosphate efficiently from synthetic wastewater. J. Biosci. Bioeng., 95, 637-640 (2003). |
| 30 | Motomura, K., Takiguchi, N., Ohtake, H., and Kuroda, A.: Polyamines affect polyphosphate accumulation in Echerichia coli. J. Environ. Biotech., 6, 41-46 (2006). |
| 31 | Lee, D., Kim, M., and Chung, J.: Relationship between solid retention time and phosphorus removal in anaerobic-intermittent aeration process. J. Biosci. Bioeng., 103, 338-344 (2007). |
| 32 | Mino, T., Van Loosdrecht, M. C. M., and Heijnen, J. J.: Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res., 32, 3193-3207 (1998). |
| 33 | Lemos, P. C., Viana, C., Salgueiro, E. N., Ramos, A. M., Crespo, J. P. S. G., and Reis, M. A. M.: Effect of carbon source on the formation of polyhydroxyalkanoates (PHA) by a phosphate-accumulating mixed culture. Enzyme Microb. Technol., 22, 662-671 (1998). |
| 34 | Satoh, H., Mino, T., and Matsuo, T.: Uptake of organic substrates and accumulation of polyhydroxyalkanoates linked with glycolysis of intracellular carbohydrates under anaerobic conditions in the biological excess phosphate removal process. Water Sci. Technol., 26, 933-942 (1992). |
| 35 | Straichan, M., Golecki, J. R., and Schon, G.: Polyphosphate-accumulating bacteria from sewage plants with different processes for biological phosphorus removal. FEMS Microbiol. Lett., 73, 113-124 (1990). |
| 36 | Wagner, M., Erhart, R., Manz, W., Amann, R., Lemmer, H., Wedi, D., and Schleifer, K. H.: Development of an rRNA-targeted oligonucleotide probe specific for the genus Acinetobacter and its application for in situ monitoring in activated sludge. Appl. Environ. Microbiol., 60, 792-800 (1994). |
| 37 | Hesselmann, R. P., Werlen, C., Hahn, D., van der Meer, J. R., and Zehnder, A. J.: Enrichment, phylogenetic analysis and detection of a bacterium that performs enhanced biological phosphate removal in activated sludge. Syst. Appl. Microbiol., 22, 454-465 (1999). |
| 38 | Garcia Martin, H., Ivanova, N., Kunin, V., Warnecke, F., Barry, K. W., McHardy, A. C., Yeates, C., He, S., Salamov, A. A., Szeto, E., Dalin, E., Putnam, N. H., Shapiro, H. J., Pangilinan, J. L., Rigoutsos, I., Kyrpides, N. C., Blackall, L. L., McMahon, K. D., and Hugenholtz, P.: Metagenomic analysis of two enhanced biological phosphorus removal (EBPR) sludge communities. Nat. Biotechnol., 24, 1263-1269 (2006). |
| 39 | Kuroda, A., Takiguchi, N., Gotanda, T., Nomura, K., Kato, J., Ikeda, T., and Ohtake, H.: A simple method to release polyphosphate from activated sludge for phosphorus reuse and recycling. Biotechnol. Bioeng., 78, 333-338 (2002). |
| 40 | Bond, P. L., Keller, J., and Blackall, L. L.: Anaerobic phosphate release from activated sludge with enhanced biological phosphorus removal. A possible mechanism of intracellular pH control. Biotechnol. Bioeng., 63, 507-515 (1999). |
| 41 | Takiguchi, N., Kuroda, A., Kato, J., Nukanobu, K., and Ohtake, H.: Pilot plant tests on the novel process for phosphorus recovery from municipal wastewater. J. Chem. Eng. Japan, 36, 1143-1146 (2003). |
| 42 | Booker, N. A., Priestley, A. J., and Fraser, I. H.: Struvite formation in wastewater treatment plants: Opportunities for nutrient recovery. Environ. Technol., 20, 777-782 (1999). |
| 43 | Stratful, I., Scrimshaw, M. D., and Lester, J. N.: Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res., 35, 4191-4199 (2001). |
| 44 | Williams, S.: Struvite precipitation in the sludge stream at slough wastewater treatment plant and opportunities for phosphorus recovery. Environ. Technol., 20, 743-747 (1999). |
| 45 | Ueno, Y. and Fujii, M.: Three years experience of operating and selling recovered struvite from full-scale plant. Environ. Technol., 22, 1373-1381 (2001). |
| 46 | Richardson, A. E.: Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust. J. Plant Physiol., 28, 897-906 (2001). |
| 47 | Whitelaw, M.: Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agronomy, 6, 99-151 (2000).. |
| 48 | Nagamine, S., Ueda, T., Masuda, I., Mori, T., Sasaoka, E., and Joko, I.: Removal of phosphorus from wastewater by crystallization on the surface of macroporous TiO2 with a fibrous microstructure. Ind. Eng. Chem. Res., 42, 4748-4752 (2003). |
| 49 | Johansson, L. and Gustafsson, J. P.: Phosphate removal using blast furnace slags and opoka-mechanisms. Water Res., 34, 259-265 (2000). |
| 50 | Ebie, Y., Kondo, T., Xu, K., Kadoya, N., Mouri, M., Maruyama, O., Noritake, S., Inamori, Y.: Recovery oriented phosphorus adsorption process in decentralized advanced Johkasou. Water Sci. Technol., 57, 1977-1981 (2008). |
| 51 | Midorikawa, I., Aoki, H., Omori, A., Shimizu, T., Kawaguchi, Y., Kassai K., Murakami, T.: Recovery of high purity phosphorus from municipal wastewater secondary effluent by a high-speed adsorbent. Water Sci. Technol., 58, 1601-1607 (2008). |
| 52 | Moriyama, K., Kojima, T., Minawa, Y., Matsumoto, S., and Nakamachi, K.: Development of artificial seed crystal for crystallization of calcium phosphate. Environ. Technol., 22, 1245-1252 (2001). |
| 53 | Schipper, W. J., Klapwijk, A., Potjer, B., Rulkens, W. H., Temmink, B. G., Kiestra, F. D., and Lijmbach, A. C.: Phosphate recycling in the phosphorus industry. Environ. Technol., 22, 1337-1345 (2001). |
| 54 | Kubo, H., Yokoyama, K., Nakajima, K., Hashimoto, S., Yamaguchi K., and Nagasaka, T: The application of material stock and flow accounting to phosphorus in Japan. J. Environ. Eng. Manage.,18: 47-53 (2008). |
| 55 | Ohtake, H., Omasa, K., and Honda, K.: Bio-based production of rock phosphate for resource recycling and environmental protection. J. Biotechnol., 136S: 5602 (2008). |
| 56 | Yamagata, Y., Watanabe, H., Saitoh, M., and Namba, T.: Volcanic production of polyphosphate and its relevance to prebiotic evolution. Nature, 352: 516-520 (1991). |
| 57 | Bork, P., Sander, C., and Valencia, A.: Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci., 2, 31-40 (1993). |
| 58 | Szymona, M. and Ostrowski, W.: Inorganic polyphosphate glucokinase of Mycobacterium phlei. Biochim. Biophys. Acta, 85, 283-295 (1964). |
| 59 | Szymona, M. and Widomski, J.: A kinetic study on inorganic polyphosphate glucokinase from Mycobacterium tuberculosis H37RA. Physiol. Chem. Phys., 6, 393-404 (1974). |
| 60 | Wood, H. G. and Goss, N. H.: Phosphorylation enzymes of the propionic acid bacteria and the roles of ATP inorganic pyrophosphate, and polyphosphates. Proc. Natl. Acad. Sci. USA, 82, 312-315 (1985). |
| 61 | Nakamura, K., Hiraishi, A., Yoshimi, Y., Kawaharasaki, M., Masuda, K., and Kamagata, Y.: Microlunatus phosphovorus gen. nov., sp. nov., a new gram-positive polyphosphate-accumulating bacterium isolated from activated sludge. Int. J. Syst. Bacteriol., 45, 17-22 (1995). |
| 62 | Tanaka, S., Lee, S. O., Hamaoka, K., Kato, J., Takiguchi, N., Nakamura, K., Ohtake, H., and Kuroda, A.: Strictly polyphosphate-dependent glucokinase in a polyphosphate-accumulating bacterium, Microlunatus phosphovorus. J. Bacteriol., 185, 5654-5656 (2003). |
| 63 | Tanaka, S., Kuroda, S., Kato, J., Ikeda, T., Takiguchi, N., Ohtake, H.: A sensitive method for detecting AMP by utilizing polyphosphate-dependent ATP regeneration and bioluminescence reactions. Biochem. Eng. J., 9, 193-197 (2001). |
| 64 | Iwamoto, S., Motomura, K., Shinoda, Y., Urata, M., Kato, J., Takiguchi, N., Ohtake, H., Hirota, R., and Kuroda, A.: Use of an Escherichia coli recombinant producing thermostable polyphosphate kinase as an ATP regenerator to produce fructose 1,6-diphosphate. Appl. Environ. Microbiol., 73, 5676-5678 (2007). |
| 65 | Sato, M., Masuda, Y., Kirimura, K., and Kino, K.: Thermostable ATP regeneration system using polyphosphate kinase from Thermosynechococcus elongatus BP-1 for D-amino acid dipeptide synthesis. J. Biosci. Bioeng., 103, 179-184 (2007). |
| 66 | Sato, M., Kirimura, K., and Kino, K.: Substrate specificity of thermostable D-alanine-D-alanine ligase from Thermotoga maritima ATCC 43589. Biosci. Biotechnol. Biochem., 70, 2790-2792 (2006). |
バナースペース
OHTAKE LAB
Biochemical Engineering Lab
Dept.of Biotechnology
Graduate School of Engineering,
Osaka University
Yamadaoka 2-1, Suita Osaka
565-0871 Japan
Phone:+81-6-6879-7437
Fax :+81-6-6879-7439